FDA to require mammogram reports include breast density information
(CNN) -- Newly updated US Food and Drug Administration regulations will require mammography facilities to notify patients about the density of their breasts.
Women with dense breasts are at higher risk for breast cancer, and dense tissue can make cancer harder to detect in mammograms. Still, few women recognize dense breasts as a significant risk factor for cancer.
The FDA update announced Thursday will also strengthen the agency's oversight over mammogram facilities, allowing it to communicate directly with patients if a facility didn't meet standards.
"Today's action represents the agency's broader commitment to support innovation to prevent, detect and treat cancer," said Dr. Hilary Marston, the FDA's chief medical officer.
About 1 in 8 women will develop breast cancer in her life, according to the US Centers for Disease Control and Prevention, and mammography remains the best tool for breast cancer screening and detection.
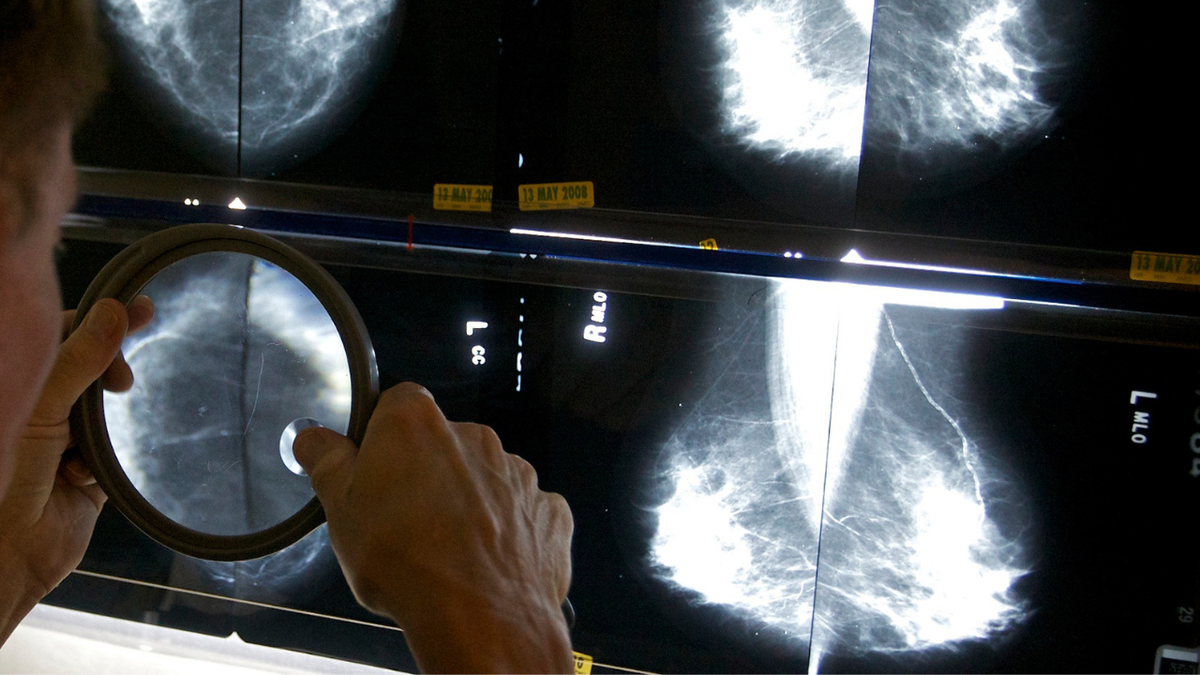
Dense breast tissue refers to breasts composed of more fibroglandular tissue than fatty tissue. It's common; the FDA says it's present in about half of women undergoing mammograms.
But it shows up as white on a mammogram, making cancer -- which also appears white -- more difficult to detect, according to the American Cancer Society. Dense breasts are also associated with up to a four times higher risk of breast cancer.
Thirty-eight states mandate that women receive written notification about their breast density and its potential breast cancer risk after they have a mammogram; however, the language varies and does not always require providers to notify a patient about their risk.
"Even though women are notified usually in writing when they get a report after a mammogram that says, 'You have increased breast density,' it's kind of just tucked in there at the bottom of the report. I'm not sure that anyone is explaining to them, certainly in person or verbally, what that means," Dr. Ruth Oratz, a breast oncologist at NYU Langone's Perlmutter Cancer Center, told CNN earlier this year.
The new changes require facilities to provide patients with information about their breast density and include specific language in the mammogram reports to explain how breast density can influence the accuracy of a mammogram.
Additionally, the FDA is requiring that facilities recommend that patients with dense breasts talk to their health care provider about breast density, risks for breast cancer and their individual situation.
"The idea is to provide information you can discuss with your provider to make better informed decisions, including if you need to consider any next steps," the FDA said in a statement.
The changes, which amend the Mammography Quality Standards Act of 1992, are required to be implemented within 18 months. The FDA says these new regulations will enhance inspection of mammography practices and communication with patients and providers.
"This is intended to help ensure important information that could affect decisions about patient care, such as the potential need for further evaluation or a repeat mammogram, is communicated as completely as possible," the agency said.
Marston says the new rules mean that "more women have access to consistent, quality mammography."