Pfizer and BioNTech seeking emergency use authorization from FDA for Covid-19 vaccine for children younger than 5
By Jamie Gumbrecht, CNN
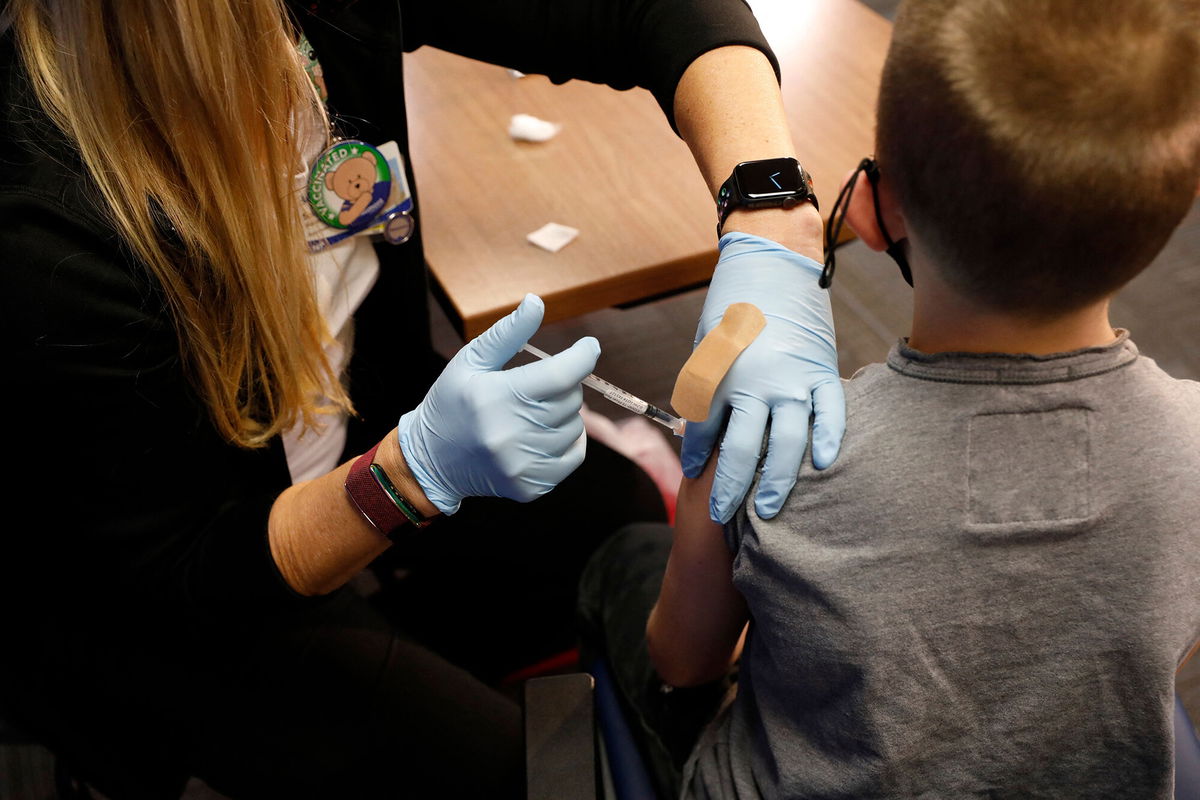
Pfizer and BioNTech are requesting emergency use authorization for their two-dose Covid-19 vaccine for children age 6 months up to 5 years, the companies said Tuesday.
Pfizer and BioNTech said they have initiated a rolling submission of data to the US Food and Drug Administration after a request from the agency. They expect to complete the EUA submission in the coming days and say they will also submit clinical trial data to the European Medicines Agency and other agencies around the world.
The FDA’s vaccine advisory committee will meet February 15 to discuss the submission.
Safety and effectiveness are key, said Dr. Paul Offit, a member of the committee and director of the Vaccine Education Center at Children’s Hospital of Philadelphia.
“The confidence of the American public depends on that, that you you’re recommending something that you would give to your own children,” Offit told CNN. “It all depends on the data. The data will tell us just how good these are. There should be a robust safety profile and a robust efficacy profile and immunogenicity profile. And if that’s true, speed doesn’t really matter, as long as they have those data.”
The Pfizer/BioNTech vaccine is already authorized for use in people as young as 5 and would be the first Covid-19 vaccine available for the youngest children.
The move comes “in response to the urgent public health need in this population,” the companies said in a news release.
Since the start of the pandemic, at least 11.4 million children have tested positive for Covid-19, the American Academy of Pediatrics reported Monday, with over 3.5 million cases reported in January alone. Children made up 22.8% of the total reported weekly cases for the week ending January 27.
“Pediatricians have seen firsthand the fear, stress and hardship that so many families of young children have endured as they await a vaccine,” Dr. Moira Szilagyi, president of the American Academy of Pediatrics, said in a statement. “We urge a transparent and data-driven process to evaluate this vaccine for this age group and look forward to offering its protection to our youngest children.”
The companies are continuing to test a three-dose version of the vaccine in the youngest kids.
In December, Pfizer extended its vaccine trial in younger children after two child-sized doses of the vaccine did not produce the expected immunity in 2- to 5-year-olds, although it did so for the babies up to age 2.
The companies said data on a third dose given at least eight weeks after the second dose is expected in the coming months, which will also be submitted to the FDA.
“As hospitalizations of children under 5 due to COVID-19 have soared, our mutual goal with the FDA is to prepare for future variant surges and provide parents with an option to help protect their children from this virus,” Pfizer Chairman and CEO Albert Bourla said. “Ultimately, we believe that three doses of the vaccine will be needed for children 6 months through 4 years of age to achieve high levels of protection against current and potential future variants.
“If two doses are authorized, parents will have the opportunity to begin a COVID-19 vaccination series for their children while awaiting potential authorization of a third dose.”
For people 12 and older, the Pfizer/BioNTech vaccine dose is 30 micrograms of vaccine, and for kids ages 5 to 11, it was stepped down to 10 micrograms. The dose for the youngest children is even lower: 3 micrograms.
CNN reported earlier Tuesday that Pfizer was encouraged to seek authorization for the two-dose vaccine by federal regulators, who hope the EUA can be granted by late February. Waiting on data for three doses could extend the wait until March.
“If the goal of the vaccine is to get baseline immunity in the kids — to prevent really bad outcomes and you’re really not using the vaccine as a tool to prevent infection in the first place — two doses could do that,” Dr. Scott Gottlieb, a former FDA commissioner and current Pfizer board member, said on CBS on Sunday. “I think that may be why federal health officials are rethinking this.”
The-CNN-Wire
™ & © 2022 Cable News Network, Inc., a WarnerMedia Company. All rights reserved.
CNN’s Katherine Dillinger, John Bonifield, Jen Christensen and Brenda Goodman contributed to this report.